Calibration and Validation Services
ICH Nominal Guidelines
Cold Chain
Bespoke Conditions
Equipment we Service
Environmental Chambers
Incubators
Refrigerators
Freezers
Ovens
Autoclaves
DI water systems
BMS / RMS systems
Photostability Cabinets
Warehouse Mapping




CALIBRATION
TESTING
CALIBRATION
Temperature - Humidity - Voltage - Resistance
We offer a fully compliant calibration service for laboratory and pharmaceutical equipment. Our services include:
- Single point Temperature Calibration
- Single point Temperature and Humidity Calibration
- PID Controller Calibration
- RTD Calibration
- Chiller Temperature Calibration
- Air Flow Calibration
All Traceable instruments are Calibrated to UKAS and NIST industry standards.

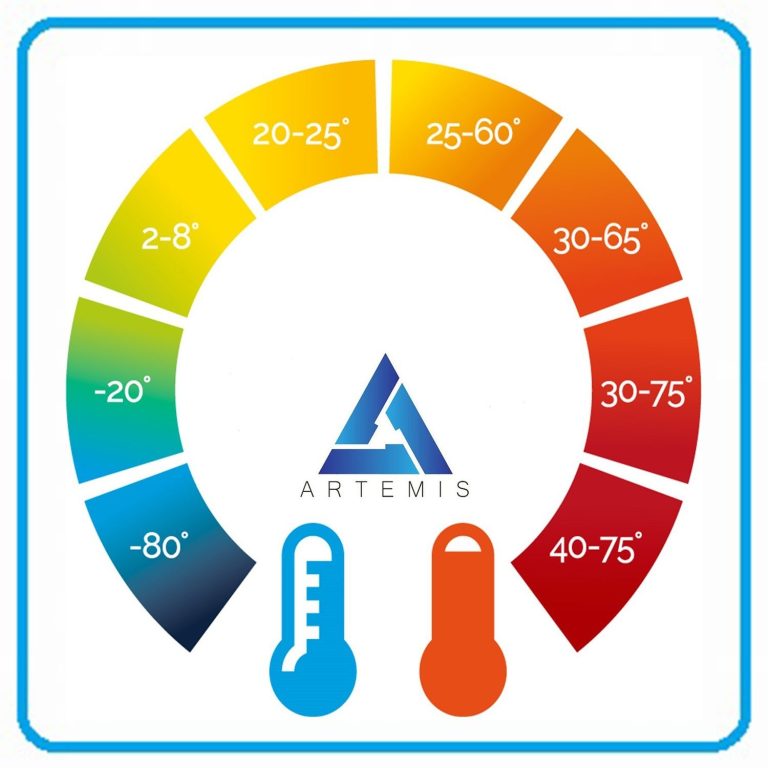
Temperature Calibration
We offer single and multi-point temperature calibration testing to UKAS and NIST industry standards for cold chain and ICH conditions. We specialize in Freezers, Fridges, Ovens, Incubators, Autoclaves, Environmental Chambers and Laboratory equipment.
-80°C, -70°C, -40°C, -20°C, 0°C, 5°C, 50°C

Temperature & Humidity
Calibration
Temperature and relative humidity calibration is available for single or multi point verification. We specialize in Freezers, Fridges, Environmental Chambers and Laboratory Equipment.
5°C, 25°C/60%RH, 30°C/65%RH, 30°C/75%RH
40°C/25%RH, 40°C/75%RH, BESPOKE
VALIDATION
TESTING
VALIDATION SERVICE
Our fully 21 CFR Part 11 compliant Data Logging system, combined with Rotonic HC2A Temperature / Humidity probes, allows us to accurately monitor and record your equipment. We provide all protocols specific to your requirements and provide charted, raw data and a full process audit trail. All documentation is completed to CGMP standards and prechecked prior to customer submission.
Conditions: 5°C, 25°C/60%RH, 30°C/65%RH, 30°C/75%RH 40°C/25%RH, 40°C/75%RH, BESPOKE
Cold Chain: -80°C, -70°C, -40°C, -20°C, 0°C
Environmental Chambers

Mapping Points
- 8 x Temperature Probes
- 8 x Temperature & 8 x Humidity
- 12 x Temperature Probes
- 12 x Temperature &12 x Humidity
- 24 Hour Unloaded Performance Test
- 1 Minute Unloaded Door Open Recovery Test
- 24 Hour Loaded Performance Test
- 1 Minute Loaded Door Open Recovery Test
- Power failure simulation Test
Refrigerators &
Freezers

Mapping Points
- 8 x Temperature Probes
- 12 x Temperature Probes
- Single Point Mapping
- Custom
- 24 Hour Unloaded Performance Test
- 1 Minute Unloaded Door Open Recovery Test
- 24 Hour Loaded Performance Test
- 1 Minute Loaded Door Open Recovery Test
- Power failure simulation Test
Ovens &
Incubators

Mapping Points
- 8 x Temperature Probes
- 12 x Temperature Probes
- Single Point Mapping
- Custom
- 24 Hour Unloaded Performance Test
- 1 Minute Unloaded Door Open Recovery Test
- 24 Hour Loaded Performance Test
- 1 Minute Loaded Door Open Recovery Test
- Power failure simulation Test
Cold Chain Chambers

Mapping Points
- 8 x Temperature Probes
- 12 x Temperature Probes
- Single Point
- Custom
- 24 Hour Unloaded Performance Test
- 1 Minute Unloaded Door Open Recovery Test
- 24 Hour Loaded Performance Test
- 1 Minute Loaded Door Open Recovery Test
- Power failure simulation Test
Photostability
Cabinets

Mapping Points
- Single Point Temperature Mapping
- UVA light saturation test
- LUX light saturation test
- PID accuracy Test
- Single point Temperature Test
- LUX Multi Point Mapping Test
- UVA Multi Point Mapping Test
- PID accuracy Test
Controlled
Warehouses

Mapping Points
- 24 x Temperature Probes
- 24 x Humidity Probes
- Single Point Mapping
- Custom
- 24 Hour Unloaded Performance Test
- 24 Hour Loaded Performance Test


We validate using a 48 channel Eurotherm 6000 series data logger. Recording real-time data using 21 CFR part 11 software.

We use Rotronic HC2A-S Temperature and Humidity probes. their reliability and test specification allows us to accurately map and document equipment performance

We specialize in testing ICH stability conditions in line with FDA , MHRA, and HPRA regulatory standards
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.